BPC-157
High-Purity Research Peptide – 10mg
BPC-157 (Body Protection Compound-157) is a rare pentadecapeptide originally traced back to gastric proteins, prized for its unusual stability and system-wide regenerative effects. Research points to powerful roles in wound closure, tendon and ligament repair, organ protection, and even driving new blood vessel growth (angiogenesis).
$75.00
BPC-157 (10mg)
BPC-157 (Body Protection Compound-157) is a synthetic peptide consisting of 15 amino acids, originally derived from a protective protein in human gastric juice. Known for its stability and resistance to enzymatic breakdown, BPC-157 has garnered attention due to its broad tissue-regenerative potential. Preclinical research suggests that these compounds play roles in accelerating wound closure, supporting tendon and ligament repair, reducing inflammation, and protecting the gastrointestinal lining.
-
Sequence: Gly-Glu-Pro-Pro-Pro-Gly-Lys-Pro-Ala-Asp-Asp-Ala-Gly-Leu-Val
-
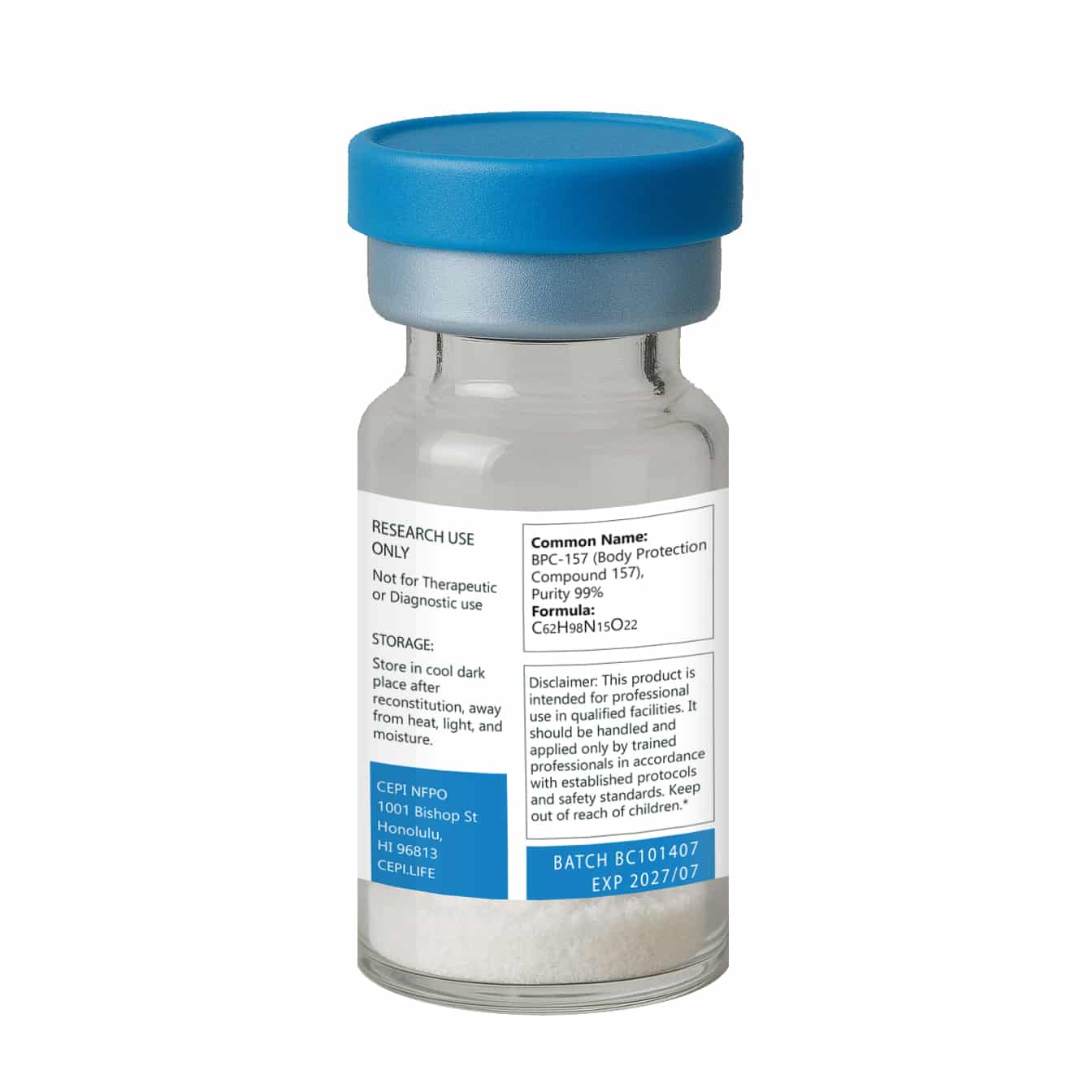
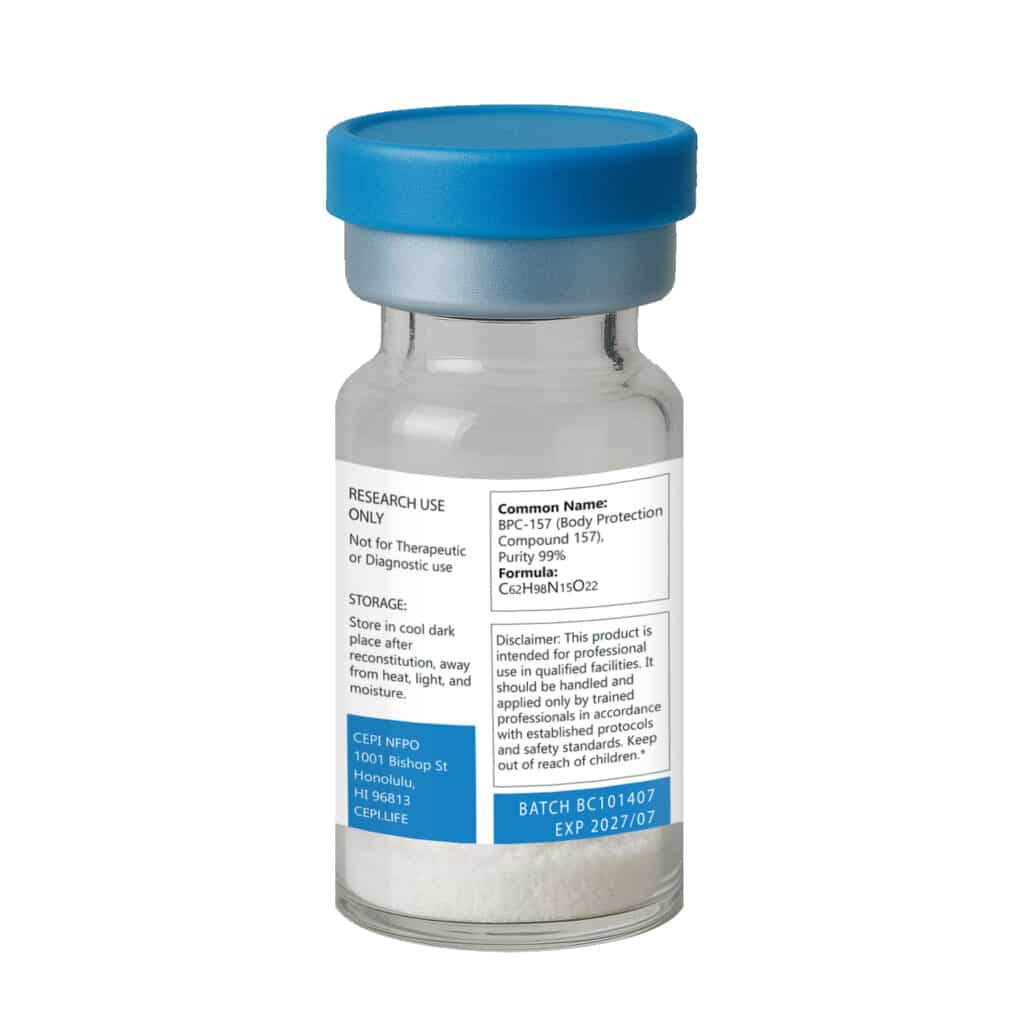
Molecular Weight: 1419.5 g/mol
-
CAS: 137525-51-0
-
Form: Lyophilized powder (no fillers)
Important: This product is sold exclusively for laboratory and in vitro research purposes. It is not intended for human consumption.
Only logged in customers who have purchased this product may leave a review.
BPC-157 has been studied across multiple domains of tissue repair and cytoprotection:
-
Wound Healing: Enhances angiogenesis, collagen synthesis, and VEGF activity, accelerating closure of incisional wounds, burns, and diabetic ulcers.
-
Musculoskeletal Repair: Promotes tendon and ligament recovery, muscle regeneration, and bone healing in animal models. Supports fibroblast activity and growth hormone receptor expression.
-
Angiogenesis & Vascular Protection: Stimulates VEGFR2-Akt-eNOS pathways, improving blood vessel formation, nitric oxide signaling, and circulation in ischemic tissue.
-
Gastrointestinal Protection: Demonstrated effectiveness in healing gastric and duodenal lesions through endothelial protection and cytoprotective effects.
-
Anti-Inflammatory Actions: Shown to reduce inflammatory responses in skin and musculoskeletal injuries, contributing to faster recovery.
While results are promising, BPC-157 research remains largely preclinical, and further studies are needed to confirm safety and efficacy in clinical settings.
References
Wound & Tissue Healing
-
Seiwerth, S., et al. (1997). BPC 157’s effect on healing. Journal of Physiology-Paris, 91, 173–178. https://doi.org/10.1016/S0928-4257(97)89480-6
-
Huang, T., et al. (2015). BPC-157 enhances alkali-burn wound healing in vivo and promotes proliferation, migration, and angiogenesis in vitro. Drug Design, Development and Therapy, 9, 2485–2499. https://doi.org/10.2147/DDDT.S82030
-
Seiwerth, S., et al. (2021). Stable Gastric Pentadecapeptide BPC 157 and Wound Healing. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.627533
Tendon, Ligament & Bone Repair
-
Chang, C., et al. (2011). Pentadecapeptide BPC 157 on tendon healing: tendon outgrowth, cell survival, and migration. Journal of Applied Physiology, 110(3), 774–780. https://doi.org/10.1152/japplphysiol.00945.2010
-
Chang, C., et al. (2014). BPC 157 enhances growth hormone receptor expression in tendon fibroblasts. Molecules, 19, 19066–19077. https://doi.org/10.3390/molecules191119066
-
Japjec, M., et al. (2021). BPC 157 therapy for disabled myotendinous junctions in rats. Biomedicines, 9. https://doi.org/10.3390/biomedicines9111547
-
Šebečić, B., et al. (1999). Osteogenic effect of BPC-157 on segmental bone defect healing in rabbits. Bone, 24(3), 195–202. https://doi.org/10.1016/S8756-3282(98)00180-X
Angiogenesis & Vascular Effects
-
Brčić, L., et al. (2009). BPC 157 modulatory effect on angiogenesis in muscle and tendon healing. Journal of Physiology and Pharmacology, 60 Suppl 7, 191–196. https://doi.org/10.1135/CSS200911118
-
Hsieh, M., et al. (2017). Therapeutic potential of pro-angiogenic BPC-157 via VEGFR2 activation. Journal of Molecular Medicine, 95, 323–333. https://doi.org/10.1007/s00109-016-1488-y
-
Hsieh, M., et al. (2020). BPC-157 modulation of vasomotor tone via Src-Caveolin-1-eNOS pathway. Scientific Reports, 10. https://doi.org/10.1038/s41598-020-74022-y
Gastrointestinal & Cytoprotection
-
Sikiric, P., et al. (1994). Protective effect of BPC-157 on gastric and duodenal lesions in rats. Life Sciences, 54(5), PL63–68. https://doi.org/10.1016/0024-3205(94)00796-9
-
Park, J., et al. (2020). BPC-157 rescues NSAID-induced cytotoxicity by stabilizing intestinal permeability. Current Pharmaceutical Design. https://doi.org/10.2174/1381612826666200523180301
-
Sikiric, P., et al. (2020). Fistulas healing with BPC 157 therapy. Current Pharmaceutical Design. https://doi.org/10.2174/1381612826666200424180139
-
Sikiric, P., et al. (2012). Focus on ulcerative colitis: stable gastric pentadecapeptide BPC 157. Current Medicinal Chemistry, 19(1), 126–132. https://doi.org/10.2174/092986712803414015
Ocular & Neurological Research
-
Masnec, S., et al. (2015). Perforating corneal injury in rat and BPC-157. The FASEB Journal, 29. https://doi.org/10.1016/j.exer.2015.04.016
-
Zlatar, M., et al. (2021). BPC-157 as therapy for retinal ischemia in rats. Frontiers in Pharmacology, 12. https://doi.org/10.3389/fphar.2021.632295
-
Sikiric, P., et al. (2023). BPC 157 as possible novel therapy for glaucoma and ocular conditions. Pharmaceuticals, 16. https://doi.org/10.3390/ph16071052
Systemic & Experimental
-
Radevski, F., et al. (2019). BPC-157 counteracts hypertension and optic disc atrophy in high-fructose diet rats. The FASEB Journal, 33. https://doi.org/10.1096/fasebj.2019.33.1_supplement.822.9
-
Šola, M., et al. (2022). Do we have a new psoriasis drug? The FASEB Journal, 36. https://doi.org/10.1096/fasebj.2022.36.s1.r5345
5-Amino-1MQ Research
5-Amino-1-methylquinolinium (5-Amino-1MQ) is a synthetic small molecule in the methylquinolinium family, defined by a quinolinium backbone with an amino group at the 5-position and a methyl group at the 1-position.
Its primary scientific interest comes from being a potent and selective inhibitor of nicotinamide N-methyltransferase (NNMT)—an enzyme involved in critical pathways linking metabolism, redox balance, and oncogenesis.
Mechanisms of Action
By inhibiting NNMT, 5-Amino-1MQ directly influences NAD+ metabolism, histone methylation, and downstream gene expression. NNMT normally consumes nicotinamide, reducing intracellular NAD+—a vital cofactor in DNA repair, energy production, and transcriptional control.
-
Preclinical adipocyte studies show that 5-Amino-1MQ raises NAD+ levels by 1.2–1.6× compared to controls, underscoring its impact on metabolic homeostasis and cellular health.
-
Epigenetic effects include altered histone methylation patterns, reshaping gene programs linked to metabolism and cancer progression.
Metabolic and Anti-Obesity Potential
Research highlights 5-Amino-1MQ as a promising metabolic modulator:
-
In diet-induced obesity mouse models, treatment significantly reduced body weight, adipose tissue mass, and adipocyte size.
-
Importantly, these benefits occurred without changes in food intake, suggesting its action comes from suppressing lipogenesis (fat creation) rather than appetite suppression.
Such findings position 5-Amino-1MQ as a candidate compound for further exploration in obesity, metabolic syndrome, and type 2 diabetes.
Applications in Cancer Research
Beyond metabolic health, 5-Amino-1MQ shows compelling potential in oncology:
-
Epigenetic remodeling: Alters histone methylation in cancer-associated fibroblasts (CAFs), disrupting tumor-supportive signaling.
-
Tumor microenvironment effects: Reduces tumor cell proliferation and modifies surrounding tissue dynamics in animal models.
These dual actions suggest possible roles for 5-Amino-1MQ in monotherapy and combination strategies, particularly in gynecological and other malignancies.
Pharmacology Insights
Early data confirms cellular permeability and bioactivity, supporting research across multiple tissue types. Ongoing studies aim to refine its pharmacokinetics and pharmacodynamics—focusing on metabolic stability, targeted delivery, and minimizing off-target effects.
References:
-
Conlon, N., & Ford, D. (2022). A systems-approach to NAD+ restoration. Biochem Pharmacol.
-
Myong, S., Nguyen, A., & Challa, S. (2024). NAD+ metabolism in gynecological cancers. Cancers.
-
Li, X., et al. (2022). NNMT as a biomarker and therapeutic target in cancer. Front Oncol.
-
Liu, J., et al. (2021). NNMT in obesity and type 2 diabetes. Biomed Res Int.















Reviews
There are no reviews yet.